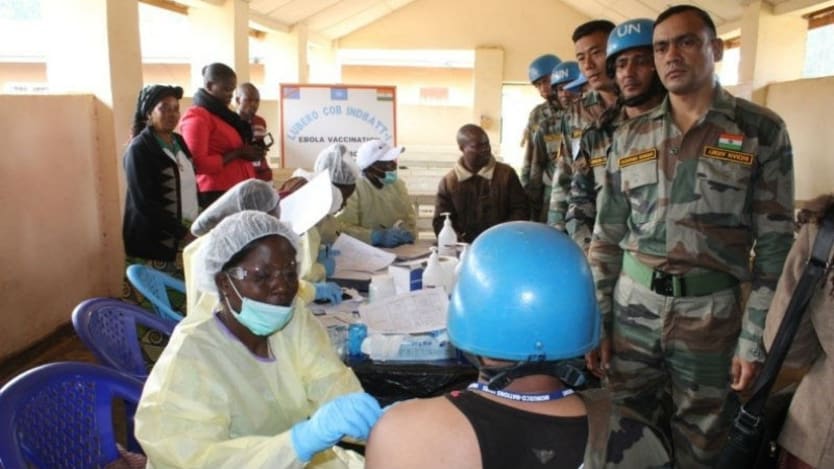
BARCELONA — Five African countries have become the first to license a highly effective Ebola vaccine, meaning it can now be administered without being subject to clinical trial or research protocols.
Regulatory bodies in the Democratic Republic of the Congo, Burundi, Ghana, Zambia, and Guinea all approved the vaccine in the past week. Several other countries are expected to do so in the coming weeks.
When the current Ebola outbreak began in DRC, a vaccine had been developed but not yet approved. The World Health Organization accelerated certification and access to it with unprecedented speed in an effort to contain the outbreak, which has already claimed more than 2,200 lives.
Ebola in DRC: NGOs organize to coordinate community engagement work
NGOs responding to the Ebola emergency in the Democratic Republic of the Congo have formed a consortium to help better involve communities.
“It’s not a treatment, it’s a vaccination but it does have an effect on the course of the disease even if you're already exposed, explained Margaret Harris, a spokesperson for WHO.
Just three months ago, the agency declared that the vaccine met quality, safety, and efficiency standards. This prequalification made it possible for African countries to start their own regulatory processes, which they did in tandem, Harris said. Once a country has licensed the vaccine, they are essentially free to decide how to use it.
The vaccine was one of five treatments approved in June 2018 by an ethics committee in DRC under a research protocol, also known as “compassionate use” or “expanded access.” Under these protocols, the drug could be administered as long as consent was obtained from the patient and monitoring protocols were followed. The vaccine has so far been issued to more than 290,000 people in DRC as well as health and frontline workers in Uganda, South Sudan, Rwanda, and Burundi.
Preliminary results indicate that the rVSV-ZEBOV-GP vaccine, an injectable manufactured by Merck, or MSD as it is known outside the United States and Canada, has been effective in 97.5% of cases and could reduce the chances of death in those already infected.
“We know that even if somebody has already been exposed, already infected, if we vaccinate them, the course of disease is far less severe [and] ... the survival rate is very, very high,” Harris said.
In DRC, the vaccine was initially delivered by WHO employees who were brought in mainly from Guinea, which is considered to be a world expert when it comes to the Ebola vaccine, Harris said — a randomized trial of the vaccine was first rolled out there during the 2015 West Africa outbreak.
The vaccination technology was then transferred to local teams who received training and played an important role in getting communities on board. The DRC Ministry of Public Health is now increasingly running these teams.
The vaccine has been a “huge game-changer,” Harris said. The downside is that people might now believe Ebola can be easily beaten. But the vaccine alone cannot eradicate Ebola and building community trust is still critical, Harris cautioned, referencing what has become one of the big challenges of the Ebola response.
It’s important to listen to communities and understand how they interpret the situation, to ensure that people who have been exposed to the disease feel safe in identifying themselves, she said.
Search for articles
Most Read
- 1
- 2
- 3
- 4
- 5