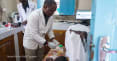
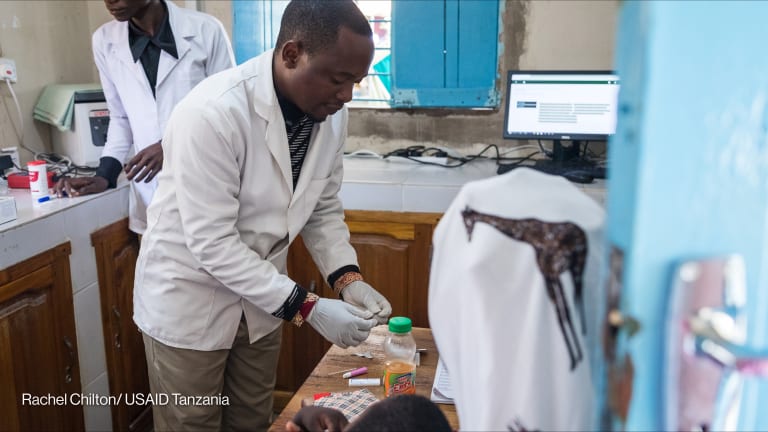
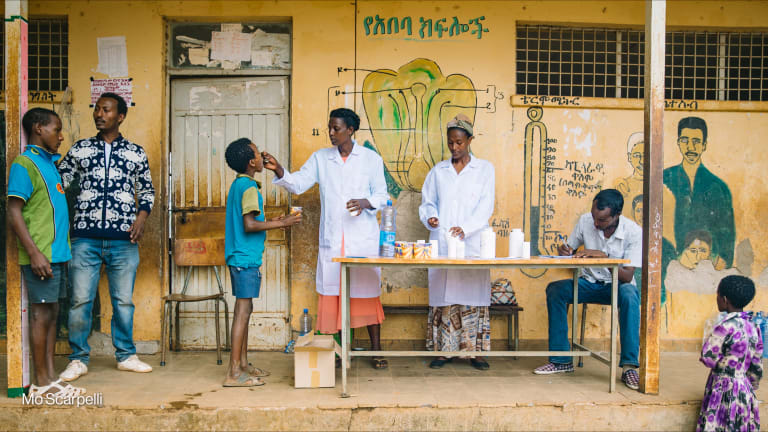
The current crisis in Syria is an extreme example of an acute need for coordinated humanitarian response. Eighteen million Syrians — 13.5 million people in Syria and 4.5 million asylum seekers and refugees outside the country — are in need of assistance, including access to medicines and vaccines.
The situation in Syria has prompted humanitarian stakeholders to re-examine the roles and potential responsibilities that different players should take in responding to major humanitarian crises. This includes the pharmaceutical industry — a significant private sector global health stakeholder.
This conversation will continue at upcoming international events such as the World Humanitarian Summit in May 2016. Indeed, WHS 2016 will focus specifically on the role of the private sector.
Timely access to essential medicines becomes especially vital in emergency situations, and pharmaceutical companies may donate medicines as a result. There is active debate around whether or not these companies should make such donations at all, due to concerns around who decides which diseases should be treated and how. This is part of a larger conversation about how to improve humanitarian (and development) efforts to actually meet local needs — we know that altruism is not enough.
While this debate goes on, many pharmaceutical companies continue to make product donations a common feature of their corporate social responsibility efforts. In response to the current crisis in Syria, for example, several major multinational pharmaceutical companies have donated medicines and cash to emergency relief organizations, including the International Committee of the Red Cross, International Health Partners and Project Hope. Other humanitarian organizations, such as Médecins Sans Frontières, generally decline to accept such donations, citing, for example, the impact on efficiency, and the potential for conflicts of interest.
Read our stories from #AcrossBorders: Origins
► DevExplains: Refugee vs. migrant
► Can we get smarter at preventing people from fleeing?
► Toward WHS 2016: The world must step up its political commitment
This raises the question: what role and what additional responsibilities should pharmaceutical companies take when donating medicines in crisis situations?
Since 2008, the Access to Medicine Index has tracked the policies and practices of pharmaceutical companies in a range of access-related activities in low- and middle-income countries, including medicine donations. We have also worked with a range of stakeholders to build consensus around what the global community can expect from pharmaceutical companies that donate medicines.
While the companies that we track tend to focus on longer-term, structured product donations that target specific diseases, particularly neglected tropical diseases (for example, ivermectin for the treatment of river blindness), the standards that apply to those programs are also important for ad hoc donations made in response to emergency situations.
At a minimum, we expect pharmaceutical companies to align with internationally accepted guidelines, such as those developed by the World Health Organization and the Partnership for Quality Medical Donations. These guidelines emphasize that recipients of donations can and should have a say as to whether the donated medicines will actually meet their needs.
There must also be no double standard in quality between medicines that are donated and those that are sold: donations must not be used as an opportunity to dispose of expired, unsafe or otherwise unwanted medicines that place a burden on health systems when they are least able to cope.
In a crisis, speed and efficiency are paramount, but must not come at the expense of these standards. To ensure that both these objectives are met, we believe that companies should have their own emergency preparedness protocols. That is, they should be alert to crises where their medicines may be needed, and have a systematic and proactive approach to engaging with organizations that are coordinating or delivering health care. When responding with medicine donations, these should be of guaranteed high quality, suitable for use, and delivered promptly.
Companies should also consider how their production capacity can meet emergency needs, to ensure ongoing and reliable supply to regular buyers as well as those affected by crises. Many companies now supply medicines and other medical supplies pre-emptively to high risk areas and humanitarian distribution hubs, in order to ease pressure on supply chains during crises.
In crisis situations, pharmaceutical companies rely on nongovernmental organizations to get medicines to the people that need them. However, the responsibility for effective last-mile delivery to patients in need is ultimately shared. For donations to be effective, companies and partners must also have a shared understanding of what is needed.
This can include transportation and logistics, trained health workers, national regulatory requirements (such as import licences) being met, and the need for additional funds to address these issues. Independent intermediaries, such as International Health Partners, that understand both the regulatory requirements and supply chain pressures of the industry, and the operating context and concerns of emergency relief organizations, are valuable in coordinating an effective industry response.
We see that the global health community, including the pharmaceutical industry, has an important role to play in clearly defining the scope of responsibility for companies in times of crisis. In the meantime, companies should have protocols in place to ensure they are ready to respond to crises such as the ongoing conflict in Syria. This will ultimately support the common goal of making effective and appropriate medicines, diagnostics and vaccines available to those that need them.
Across Borders is a monthlong online conversation hosted by Devex and partners — World Vision, the European Commission's Humanitarian Aid and Civil Protection department, the U.S. nonprofit partner of the International Organization for Migration and United Nations Volunteers — to analyze and amplify the discussion on global migration and current refugee crises through the lens of global security, development cooperation and humanitarian aid work, and more. Visit the campaign site and join the conversation on social media tagging @devex and #AcrossBorders.