NAIROBI — While controlled trials are underway to investigate the use of the most commonly used tuberculosis vaccine to protect people against COVID-19, scientists are cautioning against using it before there is evidence to prove it works, because it could lead to stock-outs that would limit access to those who need it to combat TB.
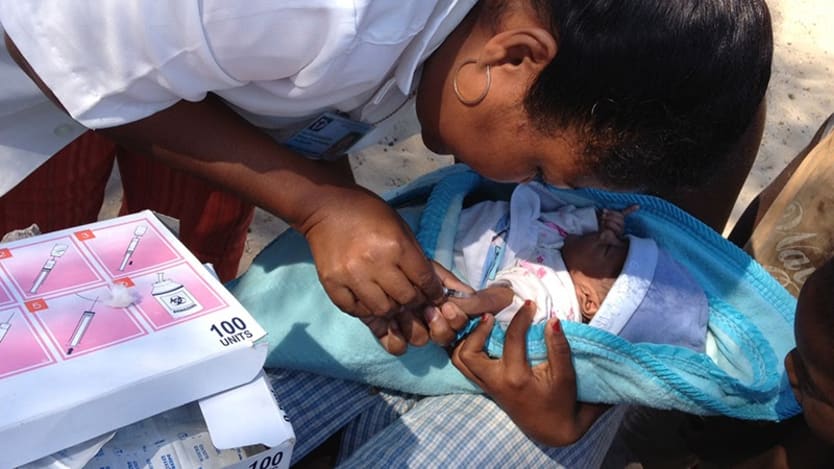
Health workers have vaccinated children with the bacille Calmette-Guérin vaccine for a century, primarily for the prevention of life-threatening forms of TB.
“Protect BCG supplies for young children.”
— The UnionResearch has also shown varying levels of efficacy in the vaccine’s ability to boost the general immune system, although no randomized control studies have yet proved this.
“These effects have not been well characterized and their clinical relevance is unknown,” according to the World Health Organization.
An observational study, not a randomized controlled study, published last month, which has not been peer-reviewed, noted that COVID-19 death rates are lower in countries that widely use the TB vaccine. In response to the study, some health workers and members of the public are requesting to be re-vaccinated with hopes of protecting themselves and others in their family.
UK offers little detail on how aid-funded COVID-19 vaccine will be accessible to all
The U.K. government has spent more than £300 million ($373 million) on treatment and vaccine research for COVID-19 but has not said how it will ensure access for lower-income countries.
“The possible effect of BCG protecting against illness and death from COVID-19 received much attention in especially the social media as a possible solution for the pandemic — a kind of ‘golden bullet,’” H. Simon Schaaf, professor and senior specialist at the Department of Paediatrics and Child Health at Stellenbosch University, told Devex in an email.
In response to this growing attention around the prospects of the use of this vaccine for COVID-19, a group of scientists, including Schaaf, published an article Tuesday in The International Journal of Tuberculosis and Lung Disease, warning that the vaccine should only be used as a prevention for TB, unless evidence proves that it is effective at preventing COVID-19. The article called for the “responsible stewardship” of the vaccine during this crisis.
About 1.5 million people died from TB in 2018 and it’s the leading cause of death globally from a single infectious agent.
The observational study provided circumstantial evidence but did not confirm causality, according to the journal article, and it was also subject to bias, including the exclusion of countries that contradict the authors’ conclusion, as well as not taking into account that countries are at different stages of the pandemic.
“The use of BCG for an unproven indication is irresponsible and may deplete BCG stocks for young children, for whom it has been proven to be a lifesaving preventive tool against TB-related morbidity and mortality,” according to the journal article, “… we implore you to protect BCG supplies for young children, for whom the vaccine offers substantial proven benefits.”
Global supply shortages of the vaccine, particularly between 2014 and 2017, “highlighted the critical need to sustain newborn BCG vaccination, especially in settings with high TB and HIV burden,” according to the article. There were situations where there were dramatic increases in the cases of TB meningitis in children when the vaccine stocked out.
While manufacturer and supply chain issues have largely resolved since then, supplies remain “fragile” because of limited global production capacity of the vaccine, the article said.
WHO also published a scientific brief this month that said that in the absence of evidence the agency does not recommend the vaccine for the prevention of COVID-19.
But there are efforts to build this evidence base through three randomized control studies trials in South Africa, the Netherlands, and Australia examining whether the vaccine could help strengthen the immune system of vulnerable populations in order to combat COVID-19, such as health care workers and the elderly.
The buzz around the use of the BCG vaccine draws parallels to the current frenzy around the use of the anti-malarial drug hydroxychloroquine for COVID-19 patients. While it's an unproven treatment lacking clinical trials to prove its efficacy, U.S. President Donald Trump has lauded it as a game-changer for patients suffering from the disease, leading states and local governments to stockpile it, and some people to self medicate, which can be deadly.
The U.S. Food and Drug Administration issued a warning last week against the use of the drug to treat COVID-19 outside a hospital or clinical trial.
Visit our dedicated COVID-19 page for news, job opportunities, and funding insights.

