West Africa Ebola vaccine trial aims to strengthen local health systems
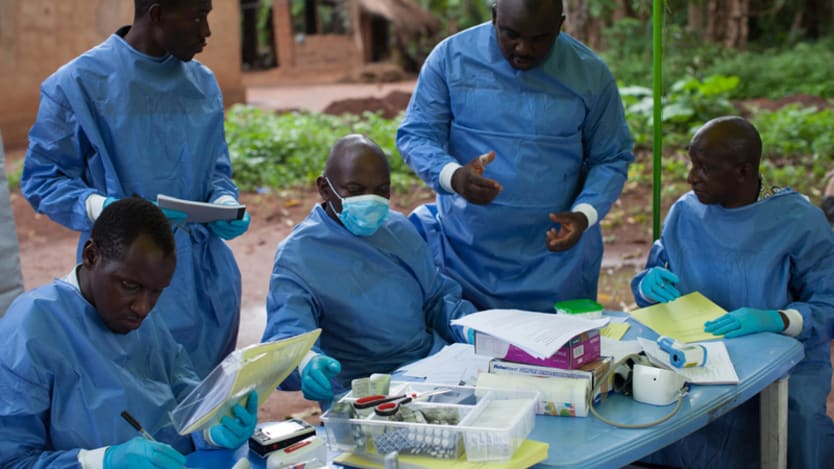
Health experts say they are confident that West African countries whose health systems were crippled by the 2014 Ebola crisis are able to conduct meaningful research in the phase two clinical trial of Ebola vaccine candidates, now underway.
The trials in Guinea, Liberia, and later Sierra Leone, are designed to strengthen health systems in those countries, as they test three vaccination strategies and two vaccines to identify which holds the most promise. A successful vaccine would need to provide enough protection from the Ebola virus to prevent, or quickly control, a future outbreak.
“Unfortunately the Ebola crisis is beyond us, but that doesn’t mean we have solved all the questions and doesn’t mean that Ebola won’t come back,” said Dr. Yazdan Yazdanpanah, principal investor for the Partnership for Research on Ebola Vaccination. “That means that the research should continue.”
A group of 5,000 adults and children will be targeted for the trial across three vaccination centers, two in Guinea (Conakry and Maferinyah), and one in Liberia (Monrovia). A test site in Sierra Leone is projected to come online later this year.
See more related topics:
▶ Q&A: Liberia's minister of health on lessons learned from the Ebola crisis
▶ Q&A: Abdelmoneim on how Ebola and armed conflict are changing MSF
The clinical trial will be supported by the French National Institute of Health and Medical Research in Guinea, by United States-based National Institute of Health in Liberia and by the London School of Hygiene and Tropical Medicine in Sierra Leone, in collaboration with local health ministries and in-country PREVAC researchers and experts.
Experts say a major focus of the trial will be on children’s immune response to the vaccines. Children tend to have a higher mortality rate than the average case fatality rate of around 50 percent, “so it is very difficult not to enroll children in the trial to evaluate the legitimacy and also the safety of the vaccine,” Dr. Yazdan said.
The trial plans to enroll 1500 children across the tested countries, with an aim to “evaluate the safety and response these vaccines elicit in children, and to determine how rapidly an immune response develops, what is the nature of the immune response and the nature of antibodies generated,” Dr. Cliff Lane, deputy director for clinical research at the National Institute of Allergy and Infectious Diseases told Devex.
Remaining questions
A ring vaccination efficacy trial conducted by the World Health Organization in 2015 reached positive conclusions on the potential efficacy of a vaccine developed by Merck Sharp & Dohme, Corp. However, the trial focused on the certain contacts of Ebola patients, leaving unanswered questions on the vaccine’s effectiveness among other populations.
In the first stage of the phase two trial, which is currently underway, researchers will evaluate a prime-boost vaccination, combining two different vaccines by Johnson & Johnson, compared to a placebo regimen. In a second stage, expected to start in the second half of 2017, the trial will evaluate three vaccination strategies, including two different strategies.
The PREVAC trial seeks to determine the rapidity, intensity and duration of immune responses generated by the various vaccination strategies. Yazdan said the trial will also study questions on how to administer the vaccine to the population as a preventative measure before an outbreak, as well as the safety of the regimen among children.
Because the West Africa region remains at risk of another Ebola flare, it is critical to determine the proper dosage in adults and children before an outbreak arrives, Lane said. “Certainly the region, in all likelihood, remains at risk of Ebola and there are a fair number of unanswered questions regarding vaccination, so we are testing these two vaccine candidates, one protocol across the three countries,” he said.
To ensure the participation of children, local community workers, social workers, anthropologists and volunteers have spent the past year informing local populations about this phase of the trial, why it’s important, what’s at stake, and how this trial could save the lives of thousands in the future.
Long term objectives
“The capacity building is a real mark and philosophy of this project … we want to train people, not only to do research, but we are focused on capacity building ... for the people.”
— Dr. Yazdan, investor for the Partnership for Research on Ebola VaccinationAlongside the immune-response goals of the clinical trial, Dr. Yazdan and Dr. Lane emphasized that the trials will strengthen the ability of each country’s health sector to treat infectious diseases and avoid future pandemics.
“The capacity building is a real mark and philosophy of this project. Through this kind of vaccine we want to train people, not only to do research, but we are focused on capacity building for the lab, capacity building for the people,” Dr. Yazdan explained.
Already fragile health systems in Liberia, Guinea, and Sierra Leone were stretched to capacity, exposing many weaknesses, during the 2014 crisis. The Partnership for Research on Ebola Vaccination in Liberia, or PREVAIL, expects to strengthen the health infrastructures to further the country’s ability to conduct clinical research.
“As we build the infrastructure, we want to be sure we are able to continue to use it to conduct meaningful research, defined by scientific rigor, interest on the part of the host country and value added to the host country,” Dr. Lane said.
By expanding the PREVAIL research program, Lane told Devex that the country will be able to study other diseases important to the region in the future, including malaria, HIV/AIDS and other illnesses.
“I certainly think there are a lot of challenges in the health care system, and I don’t know that one would distribute an Ebola vaccine as a preemptive measure to large populations,” he said. “But if there was the need for targeted immunization, I think Liberia has certainly already demonstrated their ability to do that.”
Update, June 4, 2017: This article has been updated to clarify the trial conducted by World Health Organization was a ring vaccination efficacy trial which tested a Merck vaccination.
Read more international development news online, and subscribe to The Development Newswire to receive the latest from the world’s leading donors and decision-makers — emailed to you free every business day.
Search for articles
Most Read
- 1
- 2
- 3
- 4
- 5