Victoria Amponsah was worried when she entered a clinic in Accra, Ghana, to deliver her son — she was especially distrustful of the quality of medicines at the clinic. She had heard stories and seen firsthand many other women from her village enter the clinic to give birth and not come out.
Her fears are not unfounded. UNICEF estimates that 90 percent of medicines used to treat postpartum hemorrhage in Ghana were found to be substandard and that 1 in every 68 women in Ghana will die from postpartum bleeding during childbirth.
But Victoria was lucky — when she began experiencing postpartum hemorrhaging, a complication of childbirth characterized by excessive bleeding that accounts for 30 percent of all obstetric deaths in Africa, she was given a good-quality dose of oxytocin which helps control bleeding.
Postpartum bleeding is treatable, yet Victoria’s survival and that of many others is left to chance.
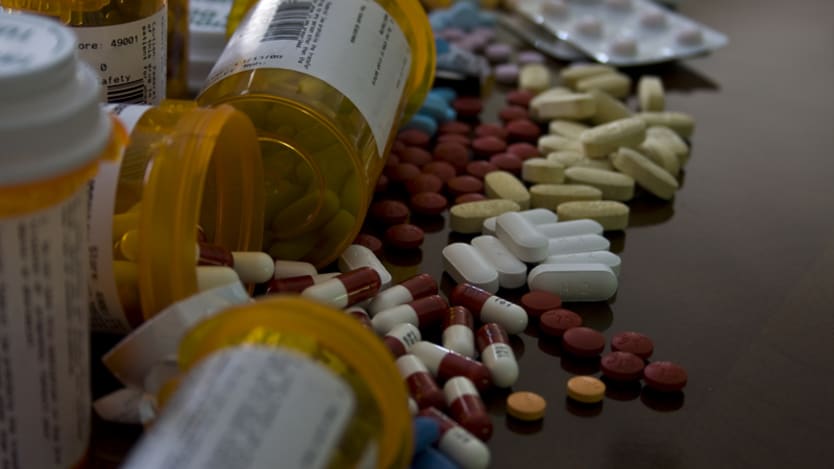
In the United States, we presume the medicines we obtain and consume, whether generic or over-the-counter, are safe and contain the correct amounts of the ingredients needed for them to work. But in many other parts of the world, no such level of trust exists. This is especially true in many resource-limited countries in Africa, where 30 to 60 percent of the lifesaving treatments available to combat the most deadly health conditions are likely to be substandard, and if used, are unlikely to fully work, according to the International Policy Network.
So what are substandard medicines? They are legal drugs that fall short of their quality specifications. Many are made, stored or distributed under poor quality conditions and contain little to none of the active ingredients needed for them to work. They can lead to drug resistance and inadequate treatment, which pose an urgent threat to vulnerable populations and jeopardize progress in combating malaria, tuberculosis, HIV/AIDS and obstetric complications like Victoria’s.
As the international community prepares to vote on the sustainable development goals, much of the discussion has been around the goal to “ensure healthy lives and promote wellbeing for all at all ages.” Working to achieve this target is commendable — but moving from aspiration to success will take a dedicated effort to eradicate the scourge of substandard drugs.
While recent attention has focused on the pervasive threats that counterfeit medicines pose, Africa’s problem of substandard medicines, one closely tied to public health and fragile health systems, is a crisis much less discussed.
These substandard drugs loom in the regions where they are needed most, including those already heavily burdened by disease and that lack access to basic necessities, such as clean water, electricity and adequate health care. Many countries lack the quality assurance safeguards and measures needed to monitor and regulate how drugs are manufactured, stored and distributed. When this happens, the medicines supply can easily become compromised, and the lives of millions of people put at greater risk.
Quality assured medicines are a pillar of public health and one underlying fundamental tenant, or inherent promise that they provide, is that they will be safe, of good quality and effective. If this promise is broken — and patients receive medicines that are substandard — then there is little hope that our treatment-based public health interventions will work.
This quality promise not only applies to pharmaceuticals, but also extends to tools used to detect, treat, and prevent disease, including vaccines, medical devices and diagnostic tests. In order for our current efforts to work, the quality of these products must be assured, not assumed.
That’s why the U.S. Pharmacopeial Convention works in countries around the world to strengthen health systems and help to secure the promise of quality that medicines should provide. We are working with the Clinton Global Initiative and other partners to help strengthen health systems in Africa and eliminate poor quality medicines from the region’s drug supply.
In 2013, we opened the Center for Pharmaceutical Advancement in Training in Ghana and made it our first commitment to action as members of the Clinton Global Initiative, where we are now addressing the unique challenges of ensuring pharmaceutical quality in sub-Saharan Africa. Through CePAT, our targeted, systems-level solutions and approaches are helping to make regulatory systems and laboratories stronger, human resources smarter, manufacturers more compliant, and the public more aware of the dangers poor-quality medicines pose.
But we are only part of a greater solution needed to increase the availability of quality-assured medicines worldwide. Sustained political will and investment of human and financial resources are also needed if we are to turn the tide.
We hope that other leaders will join us in taking actions that foster trust in the health system and strengthen the security of lifesaving drugs. The right investments can create a foundation for harvesting the instruments and resources that help ensure that successful birthing experiences are no longer considered a miracle.
Sustaining Development is a three-month online series exploring the post-2015 development agenda hosted by Devex in partnership with Chevron, FXB, Global Health Fellows Program II, Philips, Pfizer, UNIDO, U.N. Volunteers and the U.S. Council for International Business. We will look at the practical steps needed to move the sustainable development goals from concept to reality. Visit the campaign site and join the conversation using #SustainDev.
