MANILA — Experts are encouraged with the interim efficacy results of Pfizer and BioNTech’s vaccine against the coronavirus, but that optimism was tempered with concerns over the vaccine’s affordability and accessibility.
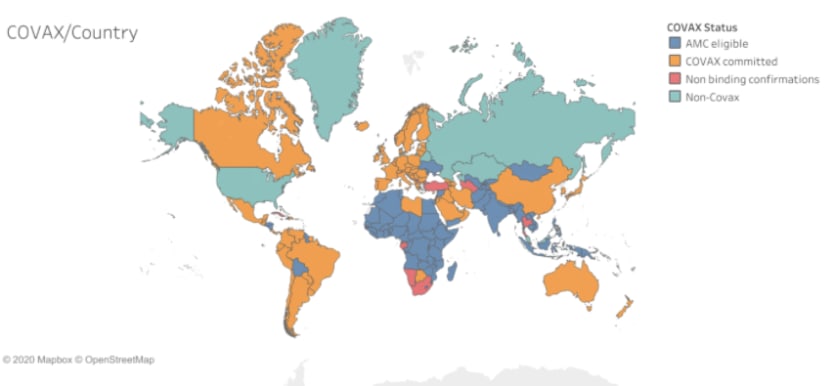
The companies also have yet to sign a deal with COVAX, the global initiative whose aim is to provide equal access to COVID-19 vaccines to low- and middle-income countries.
On Monday, Pfizer CEO Albert Bourla said in an update that the companies’ mRNA-based COVID-19 vaccine was found to be more than 90% effective in preventing the disease in people that did not have evidence of previous SARS-CoV-2 infection. Bourla described it as a critical milestone in the vaccine development work for COVID-19.
What do people in BRICS countries think about a COVID-19 vaccine?
There is a generally high acceptance of a potential COVID-19 vaccine among respondents in BRICS countries, except in Russia.
The results were from an ongoing phase 3 clinical study of the vaccine, which has enrolled 43,538 study participants. Of those, 94 have gotten COVID-19. It’s unclear how many of them received the vaccine, and how many received a placebo.
The study will continue to generate efficacy data, but also safety data from participants in the coming weeks. They are also generating data to show the vaccine can be consistently manufactured and meet quality standards. Proof of efficacy, safety, and consistent manufacturing are needed for them to be able to file for emergency use authorization from the U.S. Food and Drug Administration.
Global health leaders welcomed the news. Dr. Richard Hatchett, CEO of Coalition for Epidemic Preparedness Innovations, said the interim results “are testament to the ingenuity and skill of the scientific community in advancing vaccine candidates against COVID-19,” and increases the probability of success of other COVID-19 vaccine candidates which are using a similar approach. That includes all vaccines in CEPI’s portfolio, he said.
Gavi CEO Seth Berkley meanwhile said the data helps advance understanding of protection against the virus.
“Importance of a 1st positive signal should not be underestimated: we now know immunisation can lead to protection & that spike protein works as antigen,” he tweeted, followed by another tweet that says the organization continues to negotiate with a number of vaccine manufacturers “who share the #COVAX vision for a fair and equitable global distribution of eventual #COVID19 vaccines.”
“The vaccine will be 0% effective to the people who can’t access or afford it.”
— Niko Lusiani, senior adviser, Oxfam AmericaThere are currently 10 COVID-19 vaccine candidates in phase 3 clinical trials. But of that list, very few have entered into agreements allowing for a portion of their candidate vaccines to be made available to lower- and middle-income countries through the COVAX Advance Market Commitment. GlaxoSmithKline and Sanofi, whose vaccine is in phase 1/2 clinical trial, have signed a statement of intent to provide 200 million doses of their investigation vaccine to the COVAX Facility in late October.
Pfizer and BioNTech have not announced any formal agreements with COVAX to date. In a media briefing in September, Bourla said that they were in “very intense” discussions with Gavi, but that nothing has been concluded yet.
Devex reached out to Pfizer to ask about its COVAX commitment plans but did not receive a response before publishing.
Asked if Pfizer has already committed any number of doses of the vaccine to COVAX, a Gavi spokesperson only said that it’s commented on all formal agreements it’s had to date with companies, which include AstraZeneca, Serum Institute of India for both AstraZeneca and Novavax candidate vaccines, as well as GSK and Sanofi, adding that they “continue to discuss possible deals with several others.”
Meanwhile, Pfizer and BioNtech have already made multiple vaccine deals with a number of high-income countries. This includes an agreement with the U.S. government for 100 million doses of the vaccine, with the option to acquire up to an additional 500 million doses. They’ve also struck a deal to provide 30 million doses to the United Kingdom, 1.5 million doses to New Zealand, and 120 million doses to Japan. On Monday, Ursula von der Leyen, president of the European Commission, announced in a tweet that it will soon sign a contract with Pfizer and BioNTech for up to 300 million doses of the vaccine.
Read the COVID-19 and the Polls series:
► In India, a new election promise: A vaccine for COVID-19
► In Tanzania election, COVID-19 denialism an 'excuse to clamp down' on dissent
► Opinion: What the US election could mean for global COVID-19 vaccine access
Financial details of most of these agreements have not been made public, but in the U.S., the government will pay $1.95 billion upon receipt of 100 million doses of the vaccine, following FDA authorization or approval.
Some experts are encouraged with the latest data from Pfizer and BioNTech’s vaccine but expressed concerns about equitable access.
“Pfizer and BioNTech’s encouraging results bring us all hope that we can get out of our current pandemic nightmare, but that simply won’t happen unless the vaccine is available and affordable to everyone,” Niko Lusiani, senior adviser with Oxfam America, said in a statement.
He added that neither companies have made a public commitment to sharing COVID-19 vaccine knowledge, technology, intellectual property, data and know-how to boost vaccine supply and equity, and reduce pricing.
“The vaccine will be 0% effective to the people who can’t access or afford it,” he said.
There is also concern about the vaccine’s cold chain requirements, as it needs to be stored at very low temperatures and therefore poses a challenge for countries with inadequate cold-chain supply networks.
As Hatchett said in a statement: “Looking forwards the cold-chain requirements for this vaccine remain a challenge for use in some settings and this is something which will have to be addressed if the vaccine is to be made broadly available.”
In a news release, Pfizer and BioNTech said they expect to produce up to 50 million vaccine doses in 2020, and up to 1.3 billion doses in 2021.



