Cautious optimism: Calculating the actual value of a global health game changer

We all know that vaccines have had huge success in protecting children from deadly diseases. However, the health benefits of vaccines have not been shared equally across the globe.
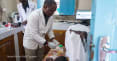
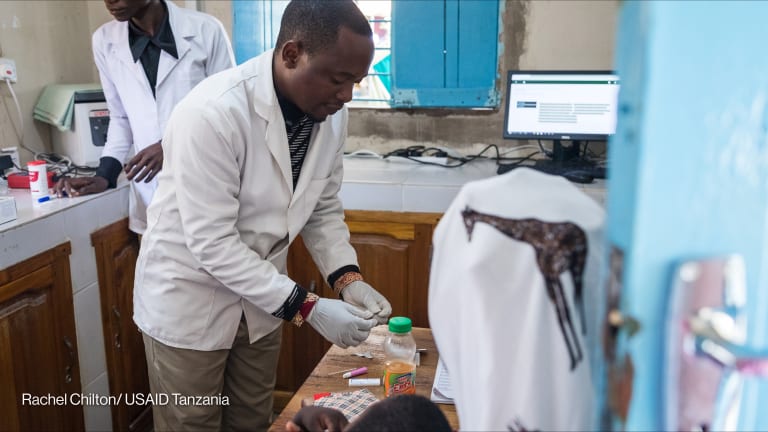
The need to keep vaccines cold in the face of high ambient temperatures and unreliable access to electricity is a challenge that limits vaccine coverage in low- and middle-income countries. The development of vaccines that are fully thermostable could increase vaccine coverage in low- and middle-income countries by enabling the stocking of vaccines at health posts that don’t have cold chain equipment, and by making outreach easier.
Fully thermostable vaccines could also increase vaccine efficacy by lowering the chance of administering vaccines impaired by heat or freeze exposure. Further, total system costs could be lowered by decreasing the cold chain footprint, vaccine wastage and vaccine delivery supply chain requirements.
As a result, the development of vaccines with greater thermostability has been thought of as a no-brainer. During the past few years, the global health community has energetically invested in improving vaccine thermostability.
Despite this, no vaccine products have been commercialized as a result.
In order to define the real value of increasing vaccine thermostability, we recently performed a study to analyze the facts.
In our study, we analyzed thermostability in the cases of specific vaccine use — such as in routine immunization or vaccine delivery campaigns — as well as in the broader context of cold chain technology and supply chain system design.
Our results surprised us.
There are important, vaccine use case-specific temporal thresholds that need to be overcome for the benefits of vaccine thermostability to be unlocked — especially for vaccines used in routine immunization. As a result, the complexities and costs needed to achieve sufficient thermostability across the routine immunization vaccine portfolio appear to be much greater than those needed for improving the vaccine supply and cold chains.
This suggests that a major effort aimed at developing and applying the technologies required to keep vaccines temperature-stable is hard to justify currently, and improving the supply and cold chains is likely to have greater impact, at least in the near to mid term.
Do our findings mean that vaccine thermostability doesn’t hold promise for helping maximize coverage and minimize costs? Of course not.
Based on our findings, we’ve formed a set of recommendations to guide the global health community in unlocking its value.
1. Define the full range of thermostability of existing vaccines, and include this information on labels, in order to enable flexible use cases such as outreach and campaign use, for instance, via migration to controlled temperature chains in specific geographies or situations.
2. Set thermostability goals — such as heat stability and freeze protection — for new vaccines during the early stages of development. This will allow full value to be harvested from vaccine thermostability using currently available technologies.
3. In the short term, focus on improving cold chain infrastructure and supply chain system design. Our analysis shows that this approach is likely to have the largest impact on total system costs and coverage in the near term — and will influence the degree of thermostability required in the future.
4. In the long term, keep monitoring for innovative technologies that could remove the entire routine immunization portfolio out of the cold chain. Dramatic improvements will require innovation, and we should welcome new ideas and technologies that could help change the game.
New global health innovations do merit celebration but seldom can they stand alone as elixirs against our toughest challenges. Rather, the true transformative power of a new investment is how well it binds with and builds on the solutions and system surrounding it.
Our study reminds us to make sure our emphasis on new approaches, such as thermostability, matches their probable impact. Our hope is that the results of this analysis will help focus research, development and delivery efforts along paths that have the greatest likelihood of facilitating the equitable public health impact of vaccines.
To read additional content on global health, go to Focus On: Innovation in partnership with Philips.
Read more stories on vaccines and immunization:
► Mountaineering, vaccine delivery and risk management
► Global partnerships key to eradicating TB
► Investments in equity will impact half a billion lives
► Equity, accountability key next steps after Gavi replenishment
Search for articles
Most Read
- 1
- 2
- 3
- 4
- 5