WASHINGTON — The director of the Centers for Disease Control and Prevention predicts that responders in the Democratic Republic of the Congo will exhaust the current supply of the Ebola virus vaccine before the pharmaceutical company manufacturing them can produce more.
“The magnitude of this outbreak is getting to the point that one has to anticipate that it will spread outside of DRC.”
— Robert Redfield, director, CDCRobert Redfield, director at CDC, relayed that warning to U.S. lawmakers on Tuesday, just as new reports indicated the number of Ebola virus cases in eastern DRC has climbed past 2,000. While the Ebola vaccine has been helpful in this unprecedentedly difficult response effort, regulatory delays have stalled production of new vaccine stocks, Redfield said in a U.S. House of Representatives hearing.
“Operationally, this isn’t really going as effectively as we’d like,” he said. “Vaccine supply is limited, and there’s a need to accelerate that supply,” he said.
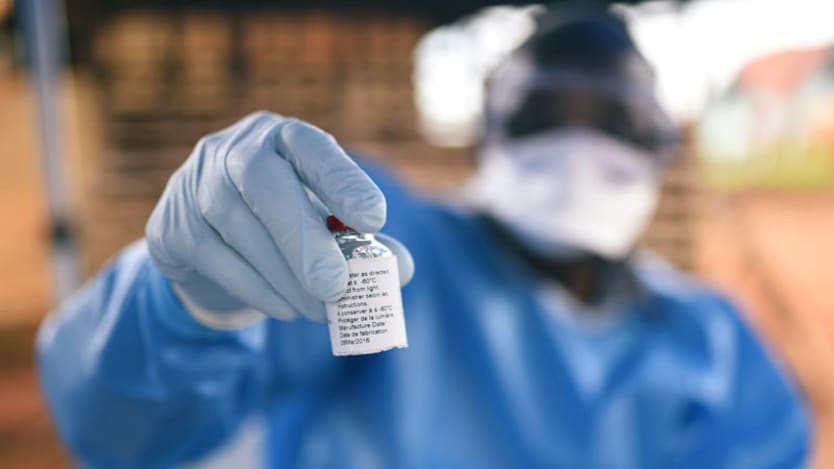
There is currently a global stockpile of about 145,000 doses of the vaccine, while roughly 130,000 people in DRC have been vaccinated so far, according to Redfield. That number of vaccinations represents only about 20% of the number of people that responders would like to be able to reach as they move toward broadening their vaccination plans, he said.
The current approach is to vaccinate people in “rings.” The first ring includes anyone in contact with someone infected with Ebola. The second ring includes anyone in contact with someone in the first ring.
“We actually want to vaccinate geographic areas where we can’t function because of the insecurity. Unfortunately, there’s going to be a six to 12-month lag before there’s adequate vaccine supply, so we do project that we are going to run out of vaccine,” Redfield said.
The problem is that the current vaccine’s manufacturer, Merck, closed its facility in Pennsylvania where the first lot of vaccines was produced. They have moved the production facility to Germany, but have been unable to secure the necessary validation for those plants that would allow production to proceed, Redfield told lawmakers.
“We’re just waiting for the validation of the new plants … The clinical efficacy data, safety data is there. It’s just waiting to prove that the plant that’s going to make the vaccine is going to make it in a reliable way,” he said.
Responders are considering a new strategy of “split dosing,” which would deliver half-sized doses of the vaccine to allow them to stretch the supply and reach more people, Redfield added.
The Merck vaccine has shown an efficacy rate of about 85%, Redfield said, though he added that in the absence of controlled clinical trials those figures represent, “back of the envelope efficacy.”
The World Health Organization has also recommended deploying another experimental vaccine developed by Johnson & Johnson.
“The magnitude of this outbreak is getting to the point that one has to anticipate that it will spread outside of DRC,” Redfield said.
Waiting on the White House
Vaccination is not the only aspect of the Ebola response that has been complicated by policy and regulatory roadblocks.
In the United States, U.S. aid officials are still waiting on their own administration to decide whether to issue a waiver that would allow additional assistance to DRC.
Under the Trump administration’s unusually strict interpretation of the Trafficking Victims Protection Act, DRC’s status as a “tier three” country in the Trafficking in Persons report has resulted in the withholding of non-humanitarian assistance to the country.
Exclusive: 'Haphazard' White House crackdown on human trafficking disrupts aid
The Trump administration's push to restrict aid to countries that are not doing enough to combat human trafficking has lawmakers worried the crackdown could impede efforts to combat Ebola.
While the president has the authority to waive these restrictions in order to protect vulnerable populations or when it is in America’s interests to do so, the White House has yet to issue a decision about DRC, according to USAID’s official in charge of the response effort, who also testified on Tuesday.
“DRC has certainly been impacted by the TVPA restrictions,” said Tim Ziemer, acting assistant administrator for the bureau for democracy, conflict, and humanitarian assistance at the U.S. Agency for International Development.
“It’s clear that additional funding would complement the current outbreak response. It would be complementary. It would build capacity. Once we get a final ruling on that, we’ll see where we stand and we’ll press ahead,” Ziemer said.
Given the deep — and sometimes deadly — distrust communities in eastern DRC exhibit towards health responders, USAID has plans to broaden its engagement in the country to include development activities that might help bridge those divides. The agency has so far struggled to explain whether those kinds of investments would even be permitted under the Trump administration’s trafficking policy crackdown.
“Not focusing resources on health, education, and community outreach hinders the success of countering the Ebola outbreak in the DRC, and I urge the administration to act more diligently now,” said Rep. Karen Bass on Tuesday.
Rep. Chris Smith, who authored the Trafficking Victims Protection Act of 2000, reiterated that the president has the authority to grant assistance waivers for countries with tier three status.
“If there is any misunderstanding with respect to how this law should be interpreted or implemented, I know that [Bass] and I would be very happy to meet with leaders of the administration to discuss that,” Smith said.








