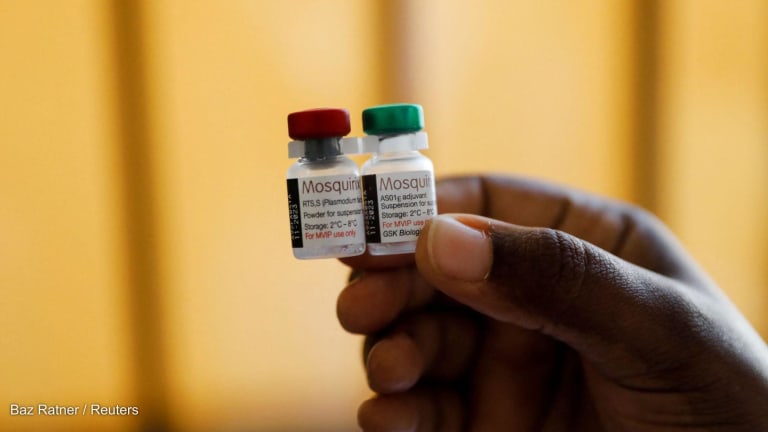
GlaxoSmithKline is granting Indian company Bharat Biotech all licensing rights to its malaria vaccine, a move that not only ensures the long-term supply of an essential tool in the fight against malaria, but also has the potential to ensure the vaccine’s accessibility and affordability for lower-income countries.
The vaccine, the world’s first vaccine for malaria, is currently being implemented in Ghana, Kenya, and Malawi, as part of a four-year pilot program to assess the feasibility of administering the vaccine and its safety as part of routine immunization in the pilot countries, as well as its potential in reducing child mortality from malaria.
More malaria news:
Just over $600M a year goes to malaria R&D. Can COVID-19 change that?
The trials and tribulations of the world's first malaria vaccine
Malaria remains a huge public health burden in many countries, but particularly in the World Health Organization’s African region, which accounted for 94% of all malaria cases and deaths globally in 2019. Children, particularly those under 5 years old, are particularly vulnerable, accounting for 67% of global malaria deaths.
GSK has committed to donating 10 million doses of the vaccine to the pilot program. If the World Health Organization recommends the vaccine for wider use, the pharma company has also committed to a supply of up to 15 million doses of the vaccine annually until the end of 2028, at manufacturing cost and no more than 5% financial return.
It’s important to ensure the vaccine is cost-effective and affordable for countries and partners eyeing to introduce the vaccine in countries after the pilot program, said John Bawa, PATH’s Africa lead for vaccine implementation.
“Once the pilot is over, and these vaccines become available, it will help accelerate the pace at which countries are able to manage and ... eliminate malaria as quickly as possible.”
— John Bawa, Africa lead for vaccine implementation, PATH“Malaria is endemic in Africa and parts of Asia, and it is important that all people who need a vaccine beyond the pilot should be able to access it, so ... barriers in terms of finance should be minimized as much as possible,” he told Devex.
Apart from licensing rights, GSK is also transferring the manufacturing capacity of the vaccine’s RTS,S antigen to the Indian biotech company. The GSK facility currently used to manufacture the RTS,S antigen will need to retire in less than 10 years. GSK however will retain production of the AS01 adjuvant, a key asset for GSK and currently used in several licensed and candidate vaccines, GSK spokesman Evan Berland told Devex in an email. But the company commits to continue supplying this key ingredient to Bharat Biotech at manufacturing cost and a financial return of no more than 5% until 2042, according to details shared with Devex via email.
The product transfer will take years due to technical, financial, and regulatory complexities in vaccine manufacturing. The full transition is expected to take place in 2029, during which time Bharat Biotech will be the sole supplier of the vaccine.
While the transfer was announced Wednesday, efforts to identify the recipient company began two years ago “to help ensure a seamless hand-off and avoid an interruption in vaccine supply,” Berland said.
Bharat Biotech was selected through a “comprehensive, competitive process” undertaken by GSK and PATH — which has been involved in the development of the malaria vaccine since 2001 as part of a public-private partnership funded by the Bill & Melinda Gates Foundation —in consultation with WHO, according to a news release. Bharat Biotech is a vaccine supplier to Gavi and UNICEF, including Rotavac for rotavirus, Typbar-TCV for typhoid fever, and Biopolio for poliovirus types 1 and 3, which are all prequalified by WHO.
Asked whether this meant GSK is moving away from malaria vaccine development amid news of the company refocusing its R&D portfolio, Berland said:
“GSK is committed to supporting global health and is focused on delivering science-led, impactful, and sustainable contributions. GSK is committed to the fight against malaria and to RTS,S – and to make its transfer to BBIL a success to allow long-term sustainable supply of the vaccine in the event of a positive WHO recommendation.”
Awaiting WHO recommendation
The Malaria Vaccine Implementation Program is expected to end in 2023, but initial data from the pilot would be available in 2021 to help WHO review and decide whether to recommend the vaccine for broader use, said PATH’s Bawa.
He said so far there have been no major causes of worry in the countries based on safety data coming from the pilot program. If this trend continues, he expects a positive recommendation from WHO on the broader use of the vaccine. But he cautioned that until WHO has fully analyzed the data, it is difficult to make a definite conclusion.
Product transfers from big pharmaceutical companies to Indian biotechnology companies aren’t entirely new, they help ensure the supply of a medical product that’s not seen as lucrative in higher-income countries, Leena Menghaney, South Asia head for MSF's Access Campaign, based in India, told Devex.
These transfers also help to have alternate manufacturers who can supply the product, and not have them at the mercy of charity, donations, or subsidized pricing from large multinational companies, she added.
But there remain outstanding questions about the malaria vaccine, including low protection and the high amount of doses, Menghaney said. These issues do not make the vaccine suitable for wide roll out in areas that need it most such as in high disease burden, low resource settings, she added.
“It’s one thing to have a vaccine, and another thing to introduce it in public health programs. The intensive resourcing required for the introduction of [the] RTS,S [vaccine] is extremely challenging, and resources could be better placed on scaling up existing malaria treatment and prevention activities,” the MSF official said.
GSK has committed supply of the vaccine until 2028 if it is recommended for wider use, but further investments will be needed to expand manufacturing capacity to meet potential projected demand for the vaccine. Bharat Biotech has initial capacity to support the transfer and the supply of the RTS,S antigen, and is planning further expansion of its vaccine manufacturing capacity, according to details shared with Devex.
However, ensuring a supply of the vaccine following a WHO approval would help tackle the burden of malaria in Africa, Bawa said.
“The clinical trials demonstrated that the vaccine can make a significant impact when added to the existing malaria intervention tools, and most African countries have an ultimate goal of eliminating malaria. So what we think is that once the pilot is over, and these vaccines become available, it will help accelerate the pace at which countries are able to manage and ... eliminate malaria as quickly as possible,” he said.
It’s still unclear what Bharat Biotech will charge for the vaccine. The company has yet to respond to Devex's request for comment. But according to an email sent to Devex, accessibility and affordability of the vaccine are among the desired outcomes of the product transfer.
“Once manufacturing at commercial scale is achieved, it will be possible to estimate the potential impact on the vaccine price. BBIL [Bharat Biotech International Limited] has committed to supply the vaccine to public sector purchasers at an affordable and sustainable price,” according to the document.