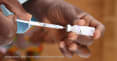
Nearly 1 in 5 people who test positive for Lassa fever in Nigeria die. The disease continues to be a disease of public health concern in many West African countries and has been on the World Health Organization’s list of priority pathogens for which there is an urgent need for accelerated research and development and countermeasures since 2016.
According to WHO, Lassa fever is endemic in Nigeria, Benin, Ghana, Guinea, Liberia, Mali, Sierra Leone, and Togo, but could exist in other West African countries.
In these countries, late detection, nonavailability of approved drugs and lack of Lassa fever-specific prevention measures continue to limit the potential of public health interventions. But an approved vaccine could be a game-changer.
Printing articles to share with others is a breach of our terms and conditions and copyright policy. Please use the sharing options on the left side of the article. Devex Pro members may share up to 10 articles per month using the Pro share tool ( ).