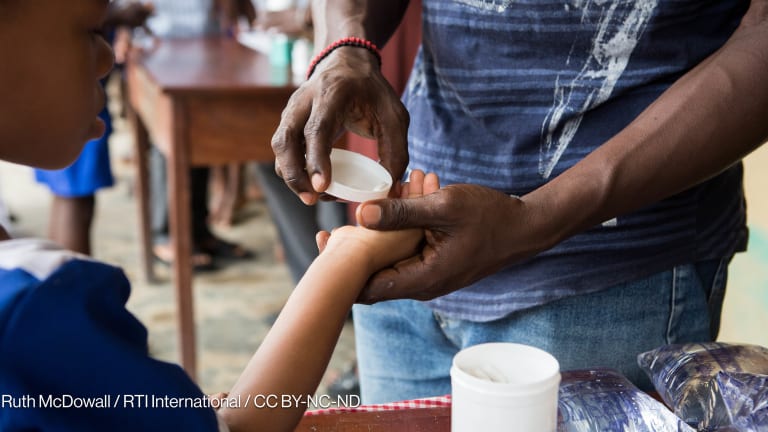
More than 30 years after the introduction of the first medicine for river blindness, a new medicine has been introduced in Africa by a nonprofit pharmaceutical company.
The Australian-based Medicines Development for Global Health said it’s the first nonprofit to bring a novel medicine to market in an endemic country for any disease without a multinational pharmaceutical or generic company.
It is now rolling out the new drug in one district of Ghana.
Printing articles to share with others is a breach of our terms and conditions and copyright policy. Please use the sharing options on the left side of the article. Devex Pro members may share up to 10 articles per month using the Pro share tool ( ).