Uganda receives first pediatric drug for schistosomiasis. What's next?
At least 50 million preschool-aged children globally are at risk of getting schistosomiasis. In the future, the new drug will be manufactured by a company in Kenya for large-scale distribution in Africa.
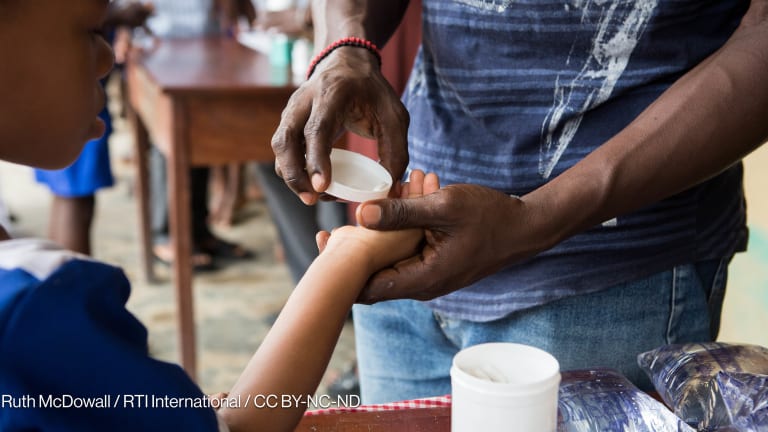
Early this month, over 2,800 preschool-aged children in Uganda were given the first preventative treatment targeted to their age group for schistosomiasis or bilharzia, a parasitic disease affecting nearly 240 million people globally. The new treatment is derived from a well-established drug used to treat schistosomiasis in school-aged children and adults. It comes in a dispersible tablet that’s palatable to preschool-aged children. At least 50 million preschool-aged children globally are at risk of getting schistosomiasis. Without treatment, schistosomiasis can affect a child’s cognitive development and cause malnutrition, anemia, and even organ damage or death. The World Health Organization has long called for children aged 2 years and above to receive preventive treatment for the disease, but no pediatric treatment option tailored for preschoolers existed until now. The Pediatric Praziquantel Consortium, which is composed of pharmaceutical companies, nonprofits, research institutes, and several schistosomiasis-endemic countries, collaborated on the drug’s development and access plans. In 2021, when clinical trials for the drug concluded, they launched a dedicated access program called ADOPT, an implementation research study to identify how delivery of the drug can be integrated into countries’ existing structures; determine the optimal means to deliver the drug in each country; and prepare for its large-scale implementation. It’s through this program that Uganda gained first access to the drug, and more pilots are planned in the country as well as in others such as Côte d'Ivoire and Kenya in the coming months. The consortium is also discussing piloting the drug in Senegal and Tanzania. “What we're hoping to have at the end of ADOPT from the work in the different countries is really kind of a toolkit and a package of materials that other countries will be able to use to then introduce the new treatment in their different contexts,” Jennifer Burrill, deputy director for access at Unlimit Health and co-lead of the ADOPT program, told Devex. Plans for scale-up Often mass treatment programs are focused on school-aged children and therefore are often delivered in schools. But the new drug is tailored and targeted at much younger children aged 3 months to 6 years who aren’t yet going to school. ADOPT aims to understand how best to reach a portion of this population, specifically those ages 2 to 5. In Uganda, they first introduced the drug through the country’s mass drug administration, but instead of schools, they opted for delivering it in the communities. Community health workers set up a station in each village where parents could bring their children for treatment. In the last few days of the campaign, the health workers went door to door to ensure they reached everyone who wanted to receive the treatment. But in the next month or so, they will be including the drug as part of a package of treatments given during child health days. This is when health workers provide a set of health care interventions for children, such as deworming, vaccinations, as well as the provision of vitamin A supplements to help boost a child’s immunity against infections. After that, they will compare the distribution coverage and acceptability between the two delivery methods to inform the best way to scale up future rollouts. They will be looking at whether similar or different delivery methods would work in other countries. In Côte d'Ivoire, for example, they are looking at testing the introduction of the drugs through the country’s nutrition programs. Through the study, Burrill said they also hope to figure out the long-term drug and funding needs to effectively integrate the drug into existing country programs. “One of the things we're trying to look at in ADOPT is what are the additional costs? Potentially the cost will be less than if there was an entirely new kind of parallel distribution structure. But that doesn't mean there wouldn't be any additional costs,” she said. Burrill said ADOPT is funding the small-scale pilots, and the second phase of the program will scale up distribution of the drug using the most promising platforms. ADOPT is funded by the European & Developing Countries Clinical Trials Partnership and the Japan-based Global Health Innovative Technology Fund. But there’s the question of how these activities will be funded in the long term. A lot of the programs they’re trialing for the drug’s distribution have components that are donor-funded. This makes the programs volatile to disruptions and changes, not to mention the dwindling funding landscape for neglected tropical disease programs. For example, data from Impact Global Health showed that research and development investments in neglected diseases were down to $3.7 billion in 2023 from $4.7 billion in 2018. Regional manufacturing in the works The drugs under the ADOPT study are being provided for free. But outside of it and for large-scale administration of the drug, it will be provided on an at cost basis, according to Johannes Waltz, board chair of the Pediatric Praziquantel Consortium and who leads Merck’s schistosomiasis elimination program. “The consortium made it very clear from the beginning … that the tablets would not be donated in order to ensure the long-term sustainability of the product,” he said. Instead, it will be open for procurement to ministries of health, implementing organizations, and multilateral agencies such as UNICEF. While some countries may require donations for some time, he said other countries, particularly in Sub-Saharan Africa are on the verge of transitioning to middle-income status, allowing them to increase their funding for public health interventions. He said there's an argument in the NTD community and wider global health to “not make countries dependent on donations.” He said the cost is still to be discussed with potential buyers and consortium partners. But he emphasized that Merck won’t profit from the drug. “We are not sort of adding through the back door the massive research and development costs which the company invested into it upfront,” Waltz said. But the drug will be manufactured closest to where the needs are. Currently, it is being manufactured by Farmanguinhos, the pharmaceutical laboratory of Fiocruz Foundation in Brazil, where schistosomiasis remains a public health problem affecting some 1.5 million people. An additional 25 million people are also living in at-risk areas of the country. But in the future, Universal Corporation Ltd. in Kenya will be the sole producer of the drug in Africa, Waltz said. “Merck is currently … going through all the processes of the technology transfer,” he said. They hope to conclude the transfer in 12-18 months. The drug received a positive scientific opinion from the European Medicines Agency in 2023, and a WHO prequalification in 2024. The consortium is now awaiting the drug’s inclusion in WHO’s essential medicines list. But while the formulation of the drug is an important milestone in the fight against schistosomiasis, eliminating the disease requires other interventions, such as people’s access to safe water and adequate sanitation. Consortium partners are also working to raise awareness and address female genital schistosomiasis, which Waltz said is “a neglected area in the context of women's health in countries which suffer from schistosomiasis.”
Early this month, over 2,800 preschool-aged children in Uganda were given the first preventative treatment targeted to their age group for schistosomiasis or bilharzia, a parasitic disease affecting nearly 240 million people globally.
The new treatment is derived from a well-established drug used to treat schistosomiasis in school-aged children and adults. It comes in a dispersible tablet that’s palatable to preschool-aged children.
At least 50 million preschool-aged children globally are at risk of getting schistosomiasis. Without treatment, schistosomiasis can affect a child’s cognitive development and cause malnutrition, anemia, and even organ damage or death. The World Health Organization has long called for children aged 2 years and above to receive preventive treatment for the disease, but no pediatric treatment option tailored for preschoolers existed until now.
This article is free to read - just register or sign in
Access news, newsletters, events and more.
Join usSign inPrinting articles to share with others is a breach of our terms and conditions and copyright policy. Please use the sharing options on the left side of the article. Devex Pro members may share up to 10 articles per month using the Pro share tool ( ).
Jenny Lei Ravelo is a Devex Senior Reporter based in Manila. She covers global health, with a particular focus on the World Health Organization, and other development and humanitarian aid trends in Asia Pacific. Prior to Devex, she wrote for ABS-CBN, one of the largest broadcasting networks in the Philippines, and was a copy editor for various international scientific journals. She received her journalism degree from the University of Santo Tomas.